EU GMP Annex 1: Are You Dressed for the Part?
What is EU GMP Annex 1?
Annex 1 is a legally binding part of the European Union Good Manufacturing Practice (EU GMP) guidelines that provides information relating to the manufacture of sterile medicinal products. The new version of Annex 1 was published on 25 August 2022 and will be implemented 1 year later, on 25 August 2023.
What Does Annex 1 Cover?
Annex 1 provides comprehensive guidance on the design, construction, and maintenance of facilities and equipment used in the manufacture of medicinal products. It also includes guidelines on production processes, quality control, and documentation.
Annex 1 applies to all sterile medicinal products manufactured in the EU and the United Kingdom (UK), as well as those manufactured elsewhere and exported into the EU and the UK. It applies to:
- Finished products
- Active substances
- Packaging materials
- Products provided in any size and combination
- Any manufacturing processes
- Any manufacturing technologies
- Any manufacturing scale (where the objective is to provide a sterile product)
- The design and control of facilities, equipment, systems, and procedures
What Are the Biggest Contamination Risks During Aseptic Processing?
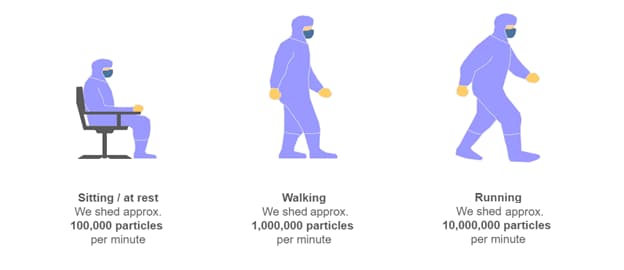
Sources of contamination within a cleanroom include raw materials, packaging, equipment, fluids, tools, processes, and, most significantly, people. Microorganisms are shed from the hair, skin, eyes and mucus membranes.1 When people move around in controlled and sterile environments, they shed 10 times more particles than when they are sitting or at rest (figure 1), hence the reason for clear guidelines on the correct controlled behavior of personnel in Annex 1 Part 7.18:
“Activities in clean areas that are not critical to the production processes should be kept to a minimum, especially when aseptic operations are in progress. Movement of personnel should be slow, controlled and methodical to avoid excessive shedding of particles and organisms due to over-vigorous activity. Operators performing aseptic operations should adhere to aseptic technique at all times to prevent changes in air currents that may introduce air of lower quality into the critical zone. Movement adjacent to the critical zone should be restricted and the obstruction of the path of the unidirectional (first air) airflow should be avoided. A review of airflow visualisation studies should be considered as part of the training programme.”
References
1 Brandes, R. Aseptic Processing: Qualification of Personnel, Mass & Peither AG-GMP Publishing, April 12, 2012.



